Now Recruiting: REACT-AF Study of Pill-in-the-Pocket Anticoagulants (Blood Thinners)
Study of Pill-in-the-Pocket Anticoagulants Uses Special AF-Sensing Apple Watch
- Summary: REACT-AF Study of Pill-in-the-Pocket Anticoagulants (Blood Thinners) is Now Recruiting
- Reading time: 3–5 minutes
People living with afib often ask if they can take blood thinners as “pill in the pocket” medications. We don’t have an answer to that since we have very little data indicating the safety or risk of doing so. It is not something you and your doctor should try. There needs to be a study of whether that is safe and effective to do.
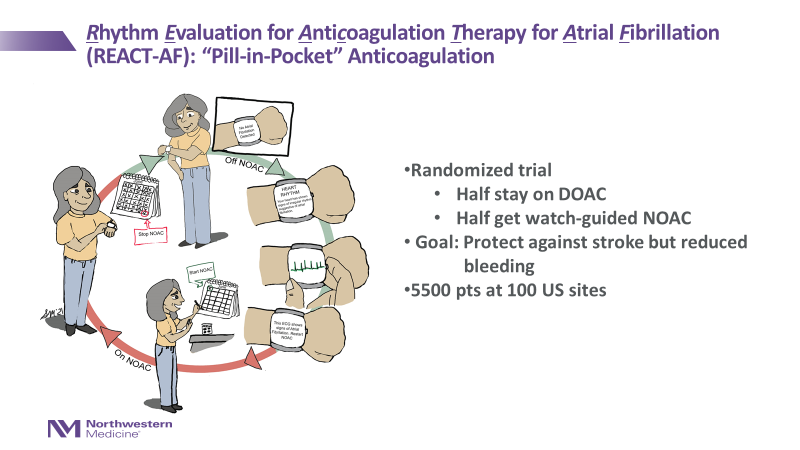
You may remember that at our 2021 Afib Patient Conference, Dr. Rod Passman shared about a proposed study called REACT-AF that would do just that.
He asked us to fill out a survey as to whether this was of interest to the afib patient community. Your interest in such a study was overwhelming and so compelling that it helped get the study funded. Dr. Passman is the Principal Investigator for this REACT-AF Study.
At the 2022 Afib Patient Conference, Dr. Marco Perez shared more about the study, and last year, Dr. Andrea Russo did so as well. This year, Dr. Jeff Healey will talk about it, too.
The study is now recruiting participants, and if you expressed interest, you may want to check it out to see if it is of interest. And if you have questions, Dr. Prystowsky, Dr. Russo, and Dr. Healey can help answer your questions at our patient conference this year.
Study Overview
The Rhythm Evaluation for AntiCoagulaTion With Continuous Monitoring of Atrial Fibrillation (REACT-AF) Study will compare continuous use of direct oral anticoagulants (DOACs) versus use of DOACs only for 30 days after an afib episode has been detected by the study’s AF-sensing Smart Watch (AFSW).
Participants will be men and women aged 22-85 with symptomatic or asymptomatic paroxysmal or persistent afib and a low-to-moderate stroke risk (CHA2DS2-VASc score of 1-4 for men, 2-4 for women).
- Participants randomized to the standard of care (control) arm will remain on previously prescribed continuous DOAC throughout the study.
- Participants randomized to the experimental arm with the study watch will take a DOAC for 30 consecutive days following a qualifying afib episode (i.e., greater than 1 hour) detected by the study watch (a special smart watch made for the study by Apple) if no further afib is detected.
Participation will last up to 60 months.
Eligibility to Participate
To participate, you should be:
- 22-85 years of age
- English-speaking
- in the US
- not in permanent afib
- have a CHA2DS2-VASC score of 1-4 for men or 2-4 for women without prior stroke or Transient Ischemic Attack (TIA)
- currently on a DOAC (direct-acting oral anticoagulant: Eliquis, Xarelto, Pradaxa, or Savaysa) and willing to stay on it or discontinue it, depending on which part of the study you are chosen for
- have an iPhone that can support the latest iOS and has a cellular service plan that you will be in range of at least 80% of the time
- be willing to wear the Apple study watch at least 14 hours a day
You are not eligible if you:
- have valvular or permanent afib
- have had afib of greater than 1 hour/month in the past three months
- are currently on warfarin and cannot take a DOAC
- are on DOAC for reasons other than afib
- are on aspirin or NSAIDS and unable to discontinue them
- have had a prior stroke or TIA
- have a pacemaker or implantable cardioverter-defibrillator
- have had an afib ablation in the past 2 months
- have had or plan to have a left atrial appendage occlusion procedure
- have a mechanical heart valve
- have hypertrophic cardiomyopathy (HCM) or other conditions listed under Participation Criteria for the study at ClinicalTrials.gov
How to Participate
To find out more and see if you are eligible, use this link to find a study site near you and reach out to the study contacts listed.
There are currently 77 study sites in the District of Columbia and 26 states (California, Colorado, Florida, Georgia, Illinois, Indiana, Iowa, Maine, Maryland, Massachusetts, Michigan, Minnesota, Missouri, New Jersey, New Mexico, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, Texas, Virginia, Washington, and Wisconsin).
To learn more about the study, see The Rhythm Evaluation for AntiCoagulaTion With Continuous Monitoring of Atrial Fibrillation (REACT-AF)
Here are recordings from our recent Afib Patient Conference talking about the REACT-AF Study. Sign in or Join our Video Library here, and then click on the links below to access these recordings:
